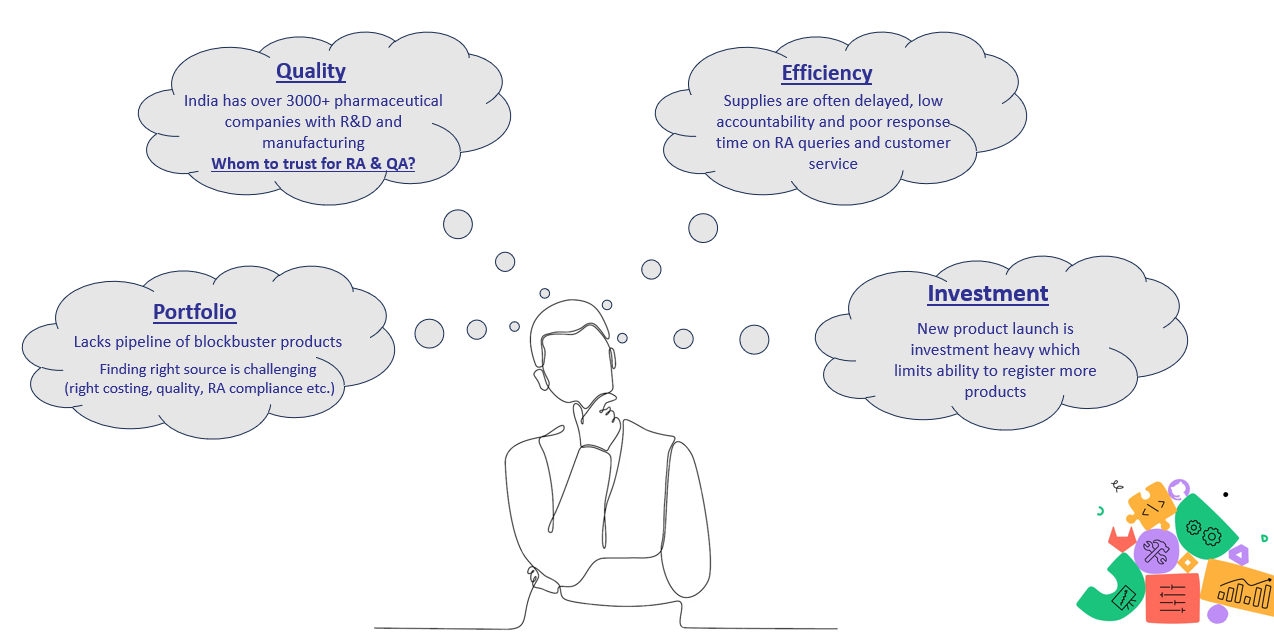
We did extensive market research to findout problem area which Zenith woud like to solve. Pharmaceutical companies in majority of countries with limited local manufacturing are marketing and distribution companies. We asked what are there common challenges ->
- Portfolio -> Ageing product portfolio and lacks pipeline. Not having blockbuster product launches. Struggling to source high quality products at affordable pricing.
- Finance -> Limited capital available hence can only take 1-2 new products annually. Plus, significant cash is blocked in tender bid bonds and paying in advance to manufacturer while receiving from MOH only in 180 days.
- Quality concerns over partnering with medium size not so known manufacturers from India -> One of our client mentioned that he partnered with 3 medium sized manufacturers but quality of first supply itself was not upto mark and now hesitant to even partner with new midsize manufacturing. Every single product is a project itself and takes significant bandwidth.
- Efficiency -> Response to RA queries was poor and supplies committed but not develivered ontime. Generally owner or senior executive from buyer side is put to interact with junior team member of manufacturer who does not have enough international exposure and lacks effective communication
How Do We Solve Each ->
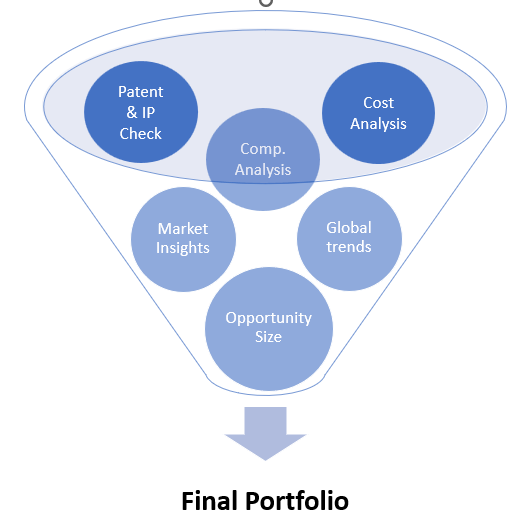
- PORTFOLIO : Our specialized portfolio team works with you to identify blockbuster products. This is done basis on global trends, IP analysis, competitor analysis, market size, growth potential, complexity to manufacture product etc. Once, we finalize products, we source these products through affiliate manufacturing partners
2. FINANCE : Co-investing in each project. We co-invest filing, registration, commercializing products thus increasing your ability to increase average launches from 1 or 2 in a year to 5 to 6 launches
3. QUALITY : We understand importance of quality.
-> Each of our manufacturing sites are EU GMP and audited by our own quality team
-> Our quality manager is present to observe batch manufacturing and quality release process
-> Finished product packs are once again inspected by our quality personnel and random sample testing by independent laboratory
4. REGULATORY : Our regulatory team reviews dossier before sharing for final submission to ensure complete dossier with minimum queries
5. EFFICIENT OPERAIONS : Strong processes of demand planning and forecasting. We only hire experienced team who understand your culture, regulatory environment and speaks your language.
Thus, we are not just your another B2B partner trying to sell product but we are your integrated partner.